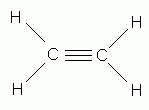
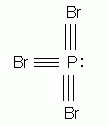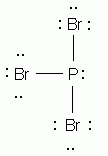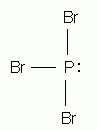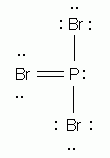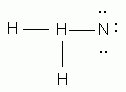In the correct Lewis structure for water, how many unshared pairs of electrons will oxygen have?
- 4
- 3
- 2
- 1
Which of the following is the correct Lewis structure for phosphorus tribromide, PBr3?
-
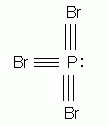
-
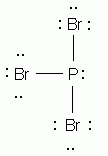
-
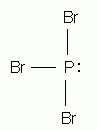
-
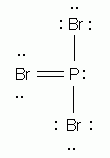
According to the HONC rule, how many covalent bonds form around nitrogen? (Spell your answer.)
Which of the following is an acceptable Lewis structure for chloromethane (CH3Cl)?
-
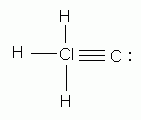
-
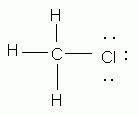
-
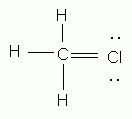
-
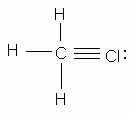
Which of the following is an acceptable Lewis Structure for the diatomic nitrogen molecule?
-
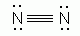
-
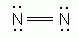
-
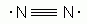
-
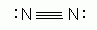
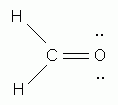
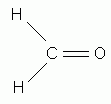
According to the HONC rule, how many covalent bonds form around oxygen? (Spell your answer.)
According to the HONC rule, how many covalent bonds form around oxygen?
-
-
-
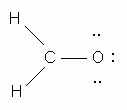
-
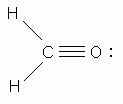
In the correct Lewis structure for the methane (CH4) molecule, how many unshared electron pairs surround the carbon?
- 2
- 8
- 4
- 0
Which of the following is NOT a valid Lewis structure?
-
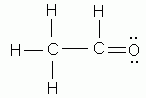
-
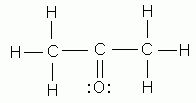
-
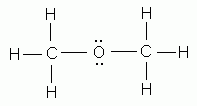
-
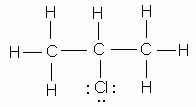
-
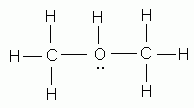
Which of the diatomic elements has a double bond between its atoms?
- hydrogen
- nitrogen
- oxygen
- flourine
According to the HONC rule, how many covalent bonds form around carbon? (Spell your answer.)
According to the HONC rule, how many covalent bonds form around hydrogen and the halogens? (Spell your answer.)
Which of the following elements will NOT be surrounded by an octet of electrons in a correctly drawn Lewis structure?
- Chlorine
- Oxygen
- Hydrogen
- Carbon
In drawing Lewis structures, a single line (single bond) between two elements represents:
- a shared pair of electrons
- an octet of electrons
- an unshared pair of electrons
- a shared electron
Which of the following is the correct Lewis structure for methanol, CH3OH?
-
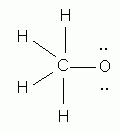
-
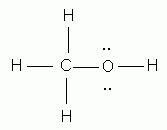
-
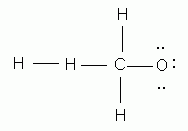
-
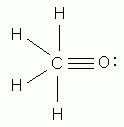
The octet rule explains the stability of most covalently bonded molecules in terms of:
- noble-gas configurations
- electron affinities
- electronegativity differences
- ionization energies
Which of the following is a correct Lewis structure for hydrogen cyanide, HCN?
-
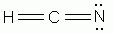
-
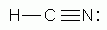
-
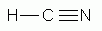
-
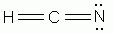
-
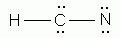
The seven elements that occur as diatomic elements are:
- H2, N2, O2, He2, Ne2, C2, Na2
- Fe2, Rn2, O2, He2, Ne2, C2, Br2
- H2, N2, O2, He2, Ne2, Cl2, Br2
- H2, N2, O2, F2, Cl2, Br2, I2
Which of the following is the correct Lewis structure for ammonia, NH3?
-
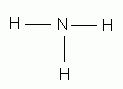
-
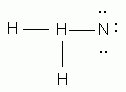
-
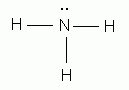
-
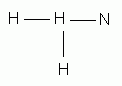
Which of the following is the correct Lewis structure for ethene (ethylene), C2H4?
-
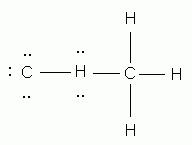
-
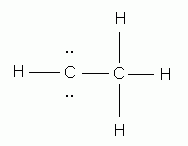
-
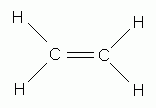
-