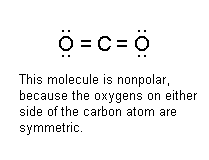All About Intermolecular Forces!
·
Try some
practice worksheets
In this helpdesk section we'll answer the following questions:
- What the heck is an intermolecular force, anyway?
- What is each type of intermolecular force and how do they work?
- How can we tell the type of intermolecular force holds molecules of a compound to each other?
- What difference does all this intermolecular force stuff make,
anyway?
|
What the heck is an intermolecular force, anyway? There are two main types of forces that can occur in a compound, intramolecular forces and intermolecular forces: Intramolecular forces are the forces that keep the atoms in a compound stuck to each other - in other words, they're just chemical bonds. For example, if you're looking at a water molecule, the force that holds the hydrogen atoms to the oxygen are intramolecular forces (covalent bonds). Same deal with sodium chloride - the forces that hold the sodium ions to the chloride ions are intramolecular forces (covalent bonds). Intermolecular forces, on the other hand,
are the forces that hold two covalent molecules to one another. For
example, if we talk about water, the forces that attract one water molecule
to another nearby water molecule are intermolecular forces. These
aren't chemical bonds like you've talked about before - instead, they're all
based on magnetic attractions between different molecules. This
attraction is caused by polarity.
|
|
What the heck is each type of intermolecular force and how do they work, anyway? There are three main types of intermolecular forces. I'll discuss each of them below, starting with the easiest one: 1) Dipole-dipole forces: In the "aside" above, we
discussed polarity. Dipole-dipole forces are simply when two molecules
that both behave like little magnets stick to each other. The strength
of the dipole-dipole force depends on how polar the molecule is, because the
more polar each molecule is, the stronger the magnetic force present.
The molecules of every polar compound are stuck to each other by these
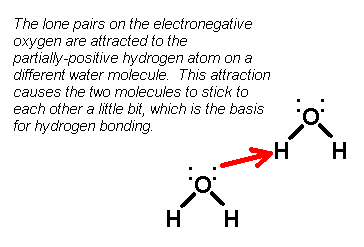
dipole-dipole forces. 2) Hydrogen bonds: Hydrogen bonds also take place in polar molecules. However, the molecules that undergo hydrogen bonding all contain a hydrogen atom that's bonded to a nitrogen atom, oxygen atom, or fluorine atom. If there are no H-N, H-O, or H-F bonds, there's no hydrogen bonding. The big question, of course, is why are hydrogen bonds different than regular dipole-dipole forces? After all, wouldn't a molecule with hydrogen bonding be polar? Of course it would! In the hydrogen-oxygen bond (or H-N or H-F bonds), the hydrogen is less electronegative than the other atom, causing the electrons in the bond to be be pulled away from it. However, in a hydrogen bond, you'll notice that the atoms that hydrogen is bonded to each have lone pairs. Because the oxygen, fluorine, and nitrogen atoms all have these lone pairs, the lone pairs tend to be attracted toward the partial-positively charged hydrogen atoms on nearby molecules. Check out the diagram below:
Hydrogen bonds are generally much stronger
than dipole-dipole forces, because the interaction of the lone pairs with the
hydrogen atoms on another molecule are very strong. 3) Van der Waals forces: Van der Waals forces are when nonpolar molecules stick together due to magnetic attractions. Now, I know what you're going to say: "Mr. Guch, only polar molecules behave like magnets. How can a nonpolar molecule have magnetic attractions? If a molecule is nonpolar, where does the magnetic charge come from? Furthermore, wouldn't this imply that nonpolar molecules are really polar? What gives?" Whoa there! Hold off on the attitude before I get angry. OK... that's better. Here's a dumb
little story to illustrate what happens.
|
|
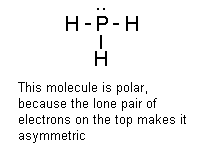
How can we tell the type of intermolecular force in a molecule? Actually, it's not that hard to figure out whether a molecule will experience hydrogen bonding, dipole-dipole forces, or Van der Waals forces. All you have to do is the following: 1) Draw the Lewis structure for the molecule. If you don't know how to draw Lewis structures, click HERE for the helpdesk section on how to do that. 2) Examine the Lewis structure and see if there's a hydrogen atom stuck directly to a fluorine, nitrogen, or oxygen atom. If it is, the molecule experiences hydrogen bonding. An important note, however, is that there may be some molecules that contain F, N, or O as well as hydrogen that aren't involved in hydrogen bonding - an example would be HCN, which has a Lewis structure that looks like H-C:::N: Remember, hydrogen bonding only takes place when the H is stuck directly to F, N, or O. 3) If the molecule doesn't have a H-N, H-F, or H-O bond, you need to keep looking. The next question: Is the molecule polar? If it is, the molecule experiences dipole-dipole forces, and if it isn't, it experiences Van der Waals forces. Of course, the big question here is this: How can you tell if a molecule is polar? Hey, I was getting to that. Basically, you can tell if a molecule is
polar by looking to see if the Lewis structure is completely symmetric.
One way you can do this is to take a look at the central atom and see if the
atoms around it are all arranged evenly. If they are, the molecule is
nonpolar. If they aren't, then it's polar. Let's see some
examples below:
Take a look at the molecule on the right. Maybe you're wondering why it's drawn like this instead of with both fluorines on opposite sides of the sulfur atom. After all, if we did that, wouldn't the molecule appear to be nonpolar? It would, and the molecule is actually polar, so that would give us a false impression. It's a general rule of thumb that if the central atom in a molecule (like the P, C, and S in the examples above) has a lone pair of electrons, the molecule is polar. I'm sure there are examples where this isn't true, but I can't think of any right now, so they can't be that common. |
|
What's all this good for, anyway? I'm glad you asked. As it turns out, intermolecular forces explain a lot of stuff about how compounds behave. For example, if a compound experiences strong intermolecular forces such as hydrogen bonding, it will have a higher melting and boiling point. This is because hydrogen bonding helps to stick the molecules together, making it harder to pull them apart. Because energy is required to make this kind of change take place, more energy in the form of heat will be needed to make hydrogen-bonded molecules melt. Another thing that intermolecular forces can do is to explain the whole "like dissolves like" thing. You've probably heard this saying, and it means that molecules with similar polarities tend to dissolve one another. If you know how to find the intermolecular forces a molecule experiences, you can figure out whether one thing will dissolve another based on their polarities. Pretty handy! Finally, it's handy because your teacher will probably ask you to come up with intermolecular forces on a test. But you already knew that, I bet. |
- To do practice worksheets about polarity, click HERE.
- To go back to the Helpdesk, click HERE.
- To go back to the Cavalcade o' Chemistry main page, click HERE.
Questions? Comments? Angry rants? Email them to me at misterguch@chemfiesta.com