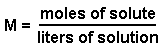So, what's the deal with solutions?
·
Try some
practice worksheets
Covered in this discussion:
- Explanation of what a solution is.
- Molarity: What it is and how to calculate it.
- Colligative properties: What they are and why we
should care.
|
Explanation of what a solution is Whenever you dissolve something in a liquid, you've made a solution. Another way of thinking about it is that if you have a liquid and it's not a pure substance, it's a solution. The liquid part is the "solvent" and the stuff you dissolved (usually a solid) is the "solute". An example: If you dissolve salt in water, the salt is the solute and the water is the solvent. A handy rule of thumb: If somebody asks you to tell you what the solvent in a solution is and you have no idea, say "water". Water is by far the most common solvent for solutions that you're likely to run into. When we're talking about how much of the solute is dissolved in the liquid, we're talking about the concentration. There are four terms we can use to describe the concentration of a solution:
|
|
Molarity: What it is and how to calculate it Molarity is one of those terms that people like to talk about a lot when describing solutions. Unlike "unsaturated", "saturated", and "supersaturated", molarity is a numerical way of saying exactly how much solute is dissolved in a solvent. As you can probably tell from the section before, the term unsaturated or supersaturated can be applied to a wide variety of solution concentrations. OK. Here's the definition of molarity: Molarity is equal to the moles of solute divided by the liters of solution. To put it in the form of an equation:
OK. Quit freaking out. It's not that hard to figure out. Let's do an example: Example: What's the molarity of a solution that contains 5.5 moles of sodium chloride in 10.5 liters of solution? Answer: M = moles / liters, or (5.5 moles) / (10.5 liters) = 0.52 M. In this case, the unit is
M. M stands for "molarity". A 0.52 M solution is
referred to as being a "0.52 molar" solution. That's simple
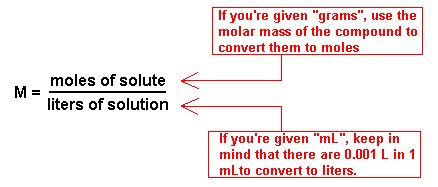
enough! Sometimes these problems get a little bit harder. Instead of giving you moles, they give you grams. Instead of liters, they give you milliliters. Fortunately, you know how to do the necessary conversions. Here's a handy diagram to help you:
Let's do an example. Example: If I have 3.50 grams of sodium chloride in 1250 mL of a solution, what's the molarity? Solution: To find molarity, we need to convert grams to moles and milliliters to liters. To convert grams to moles, we first need to divide the number of grams by the molar mass of sodium chloride. (3.5/58.5) = 0.060 moles. To convert milliliters to liters, multiply by 0.001. (1250 x 0.001) = 1.25 liters. In the final step, divide the number of moles by the number of liters to get the molarity. Since 0.060 / 1.25 = 0.048, the molarity of the solution is 0.048 M. You are now a molarity expert! |
|
Colligative properties Not surprisingly, when you dissolve something in a liquid, it has different properties than the pure liquid. Any properties that change when the concentration of a solution changes are called colligative properties. People always list boiling point elevation, melting point depression, and osmotic pressure as the main colligative properties of liquids. We're going to do it a little differently - after all, I'm not trying to sell you guys a textbook, so I don't have to use the confusing words that school districts like to see. Think about Kool Aid. Let's make a weak Kool Aid solution by dissolving one grain of Kool Aid in a glass of water. Let's also make a strong Kool Aid solution by dissolving a cup of Kool Aid powder in a glass of water. The properties that are different between the two glasses of Kool Aid are "colligative properties". In our example, we would find that:
Now that you know what colligative properties are, let's look at the ones that your textbook probably focuses on: Boiling point: As the molarity of a solution increases, the boiling point increases. This is because the solute lowers the vapor pressure of the solution and solutions don't boil until the vapor pressure of the solution is equal to atmospheric pressure. If this doesn't make any sense, just remember that strong solutions boil at higher temperatures than weak ones. Melting point: As the molarity of a solution increases, the melting point decreases. This is because the solute keeps the solvent molecules from forming a nice solid lattice. Think of this: Ocean water doesn't freeze very easily - this is because it's got salt dissolved in it. Osmotic pressure: You should have heard about this one in your biology class somewhere along the road. Basically, if you've got a solution that's separated from a pure solvent by a semi-permeable membrane, the pure solvent likes to move across the membrane to decrease the concentration of the solution. The pressure that the solvent pushes across the membrane with is called osmotic pressure. Not surprisingly, the more concentrated the solution, the more the pure solvent likes to push across the membrane and the higher the osmotic pressure. |
OK. That's it for solutions. I
hope this has helped. If so, tell all your friends to come
visit and learn about solutions. If not, then keep it to yourself because
I don't need the bad publicity. If you have questions, email me at misterguch@chemfiesta.com.
You can even ask me stupid questions if you'd like - I may laugh when I read
them, but I'll still email you back. I'm that kind of guy. If you
email me your homework questions, I'll know and make fun of you in my
response. I'll still email you, though.