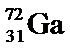Structure
of Atoms: Practice
Name_______________________________
Period_____________
Date _____________
|
Symbol |
Name |
Atomic Number |
Mass Number |
Number of protons |
Number of neutrons |
Number of electrons |
|
|
|
|
|
|
|
|
|
|
Cobalt-58 |
|
|
|
|
|
|
|
|
28 |
58 |
|
|
|
|
|
|
|
|
33 |
40 |
|
|
|
|
|
|
|
|
|
|
|
Rhodium-103 |
|
|
|
|
|
|
|
|
78 |
|
|
117 |
|
|
|
|
|
118 |
|
|
50 |
|
|
|
|
|
|
84 |
60 |
|
|
|
|
1 |
|
|
|