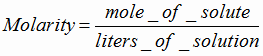 Worksheet Molarity
Worksheet MolarityName________________________________ Date ___________ Period______
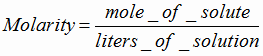 Worksheet Molarity
Worksheet Molarity
1. What is the molarity of a 2.0L solution that was made up with 4.0 moles of NaCl?
2. How many liters of solution are needed to make a 0.5M solution with 2.0 moles of BaCl2?
3. How many moles of Na2CO3 are there in 10.0 L of 2.0M solution?
4. How many moles of Na2CO3 are in 10.0 mL of a 2.0M solution?
5. If there are 10 moles of Benzene (C6H6) in a 2.5M solution. How many liters is the solution?
6. 71.5g of AgCl (MW=143amu) are dissolved in 2L of NH3 (ammonia). What is the molarity of the solution?
7. 4 moles of HNO3 (Nitric Acid) are dissolved in water to 5L. What is the molarity of the solution?
8. 6 moles of Potassium Permanganate (KMnO4) are dissolved in water to make a 3M solution. How many milliliters of solution is there?
9. How many moles of NaCl are contained in 100.0 mL of a 0.20M solution?
10. What mass (in grams) of NaCl would be contained in 500mL of 0.5M solution?
11. What volume (in mL) of 12.0 M HCl is needed to contain 3.00 moles of HCl?
12. How many moles of KCl are needed to make 100.0 mL of 0.250M solution?
13. Seawater contains roughly 28.0 g of NaCl per liter. What is the molarity of sodium chloride in seawater?
Parts per million= grams of solute x 1,000,000 grams of solution
1. What is the concentration (in ppm), of 5.0 x 10-5g chlorine molecules in 100g of pool water?
2. What is the concentration (in ppm), of 0.002g arsenic in 3000g of water?
3. What is the concentration (in ppm), of 0.05g of lead in 10g of water?
4. What is the concentration (in ppm), of 0.20g of salt in 10,000g of sea water?
5. What is the concentration (in ppm), of 0.025g antimony in 100,000g of water?
6. What is the concentration (in ppm), of 0.1 moles of hydrochloric acid molecules in 100g of rain water?